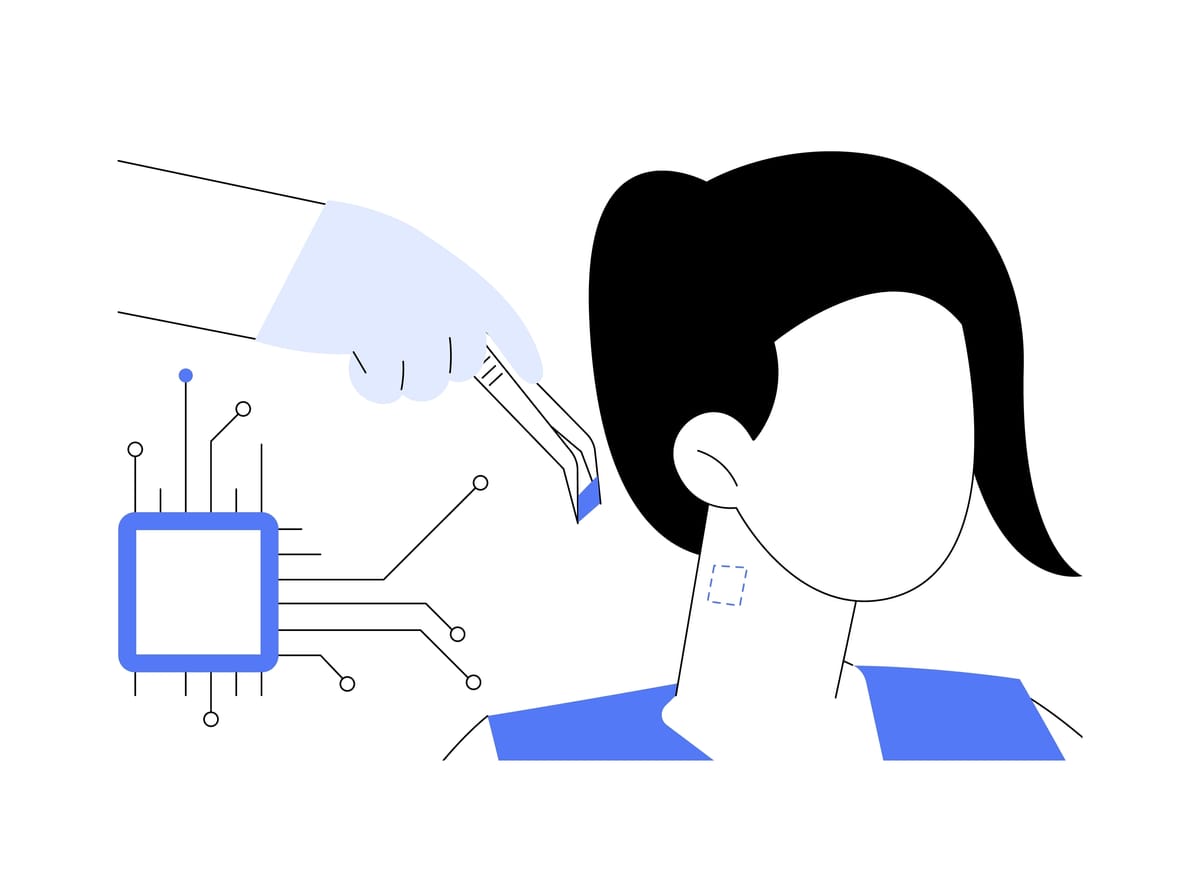
A comprehensive list of companies and products advancing neurotechnology.
Neuralink
Description
Neuralink is a neurotechnology company founded by Elon Musk and others in 2016. The company is developing ultra-high bandwidth brain-machine interfaces to connect humans and computers. Their goal is to create devices that can be implanted in the human brain to restore sensory and motor function and treat neurological conditions.
Key Metrics
- Founded: 2016
- Headquarters: Fremont, California, USA
- Founder(s): Elon Musk and others
- CEO: Elon Musk
- Employees: 250-500 (as of 2024, approximate)
- Funding: Over $680 million (as of 2023)
- Latest Valuation: Estimated at $5 billion (as of 2023)
- Key Product: Brain-computer interface chip (currently in development)
- Research Focus: Neural recording and stimulation, robotics, materials science
- Notable Milestone: First human brain implant in January 2024
Additional Reading


Synchron
Description
Synchron is a neurotechnology company focused on developing brain-computer interface technology for medical applications. Their flagship product, the Stentrode™, is a minimally invasive, endovascular brain-computer interface designed to enable patients with severe paralysis to control digital devices through thought. Unlike some competitors, Synchron's approach doesn't require open brain surgery.
Key Metrics
- Founded: 2016
- Headquarters: New York City, New York, USA (with operations in Australia)
- Founder(s): Thomas Oxley, Nicholas Opie
- CEO: Thomas Oxley
- Employees: ~100-200 (as of 2024, approximate)
- Funding: Over $145 million (as of 2022)
- Latest Valuation: Not publicly disclosed
- Key Product: Stentrode™ (brain-computer interface)
- Research Focus: Endovascular BCI, neural diagnostics, neuroprosthetics
- Notable Milestones:
Additional Reading