Synchron recently completed the COMMAND trial and achieved the first U.S. implantation of their BCI device. This article details how these milestones are transforming the lives of patients with severe paralysis.
Key Takeaways
- Synchron's COMMAND trial has successfully demonstrated the safety and efficacy of its brain-computer interface (BCI) technology in enabling independence for patients with severe paralysis.
- The unique Stentrode system allows for the minimally invasive implantation of the BCI device through the jugular vein, significantly reducing risks associated with traditional brain surgeries.
- Patient experiences from clinical trials showcase the potential of Synchron's BCI technology in improving quality of life by enabling users to control digital devices and communicate using only their thoughts.
Synchron's Command Trial Completion
The COMMAND trial, a pivotal step in brain-computer interface (BCI) advancement, was recently concluded with six participants. These individuals, all who have severe paralysis, were equipped with the Synchron Switch BCI device—a motor neuroprosthesis—under the auspices of the BRAIN Initiative by the National Institutes of Health. The endeavor occurred in multiple clinical settings and reflects a cooperative approach to this innovative work.
In assessing safety and functionality, Synchron's COMMAND trial tested whether its BCI technology allows patients enduring significant mobility limitations to carry out daily tasks independently. This investigation sought evidence that people impaired by severe movement restrictions could gain improved autonomy over digital device interaction via the Synchron Switch, potentially elevating their living standards.
Finalizing this phase confirms Synchron's technological promise and lays the groundwork for future progress within neuroprosthetic sciences.
First U.S. Implantation of Synchron's BCI Device
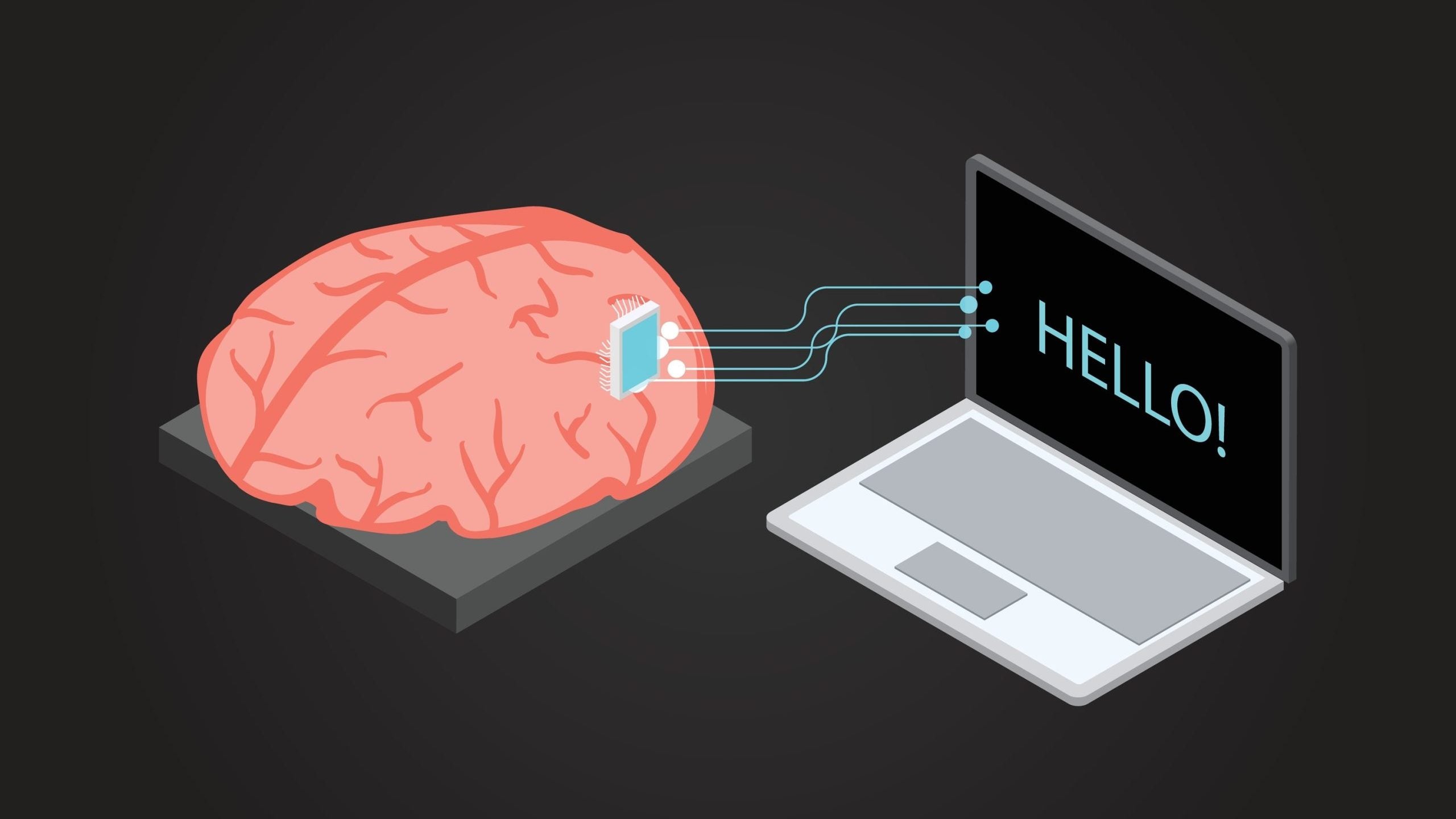
Synchron has recently celebrated a significant milestone by successfully integrating its brain-computer interface (BCI) technology into a patient in the United States for the first time. This innovative method involves gently accessing the motor cortex via the jugular vein rather than opting for traditional surgical techniques, which can be high-risk and require lengthy recovery times. By using an endovascular route, this Stentrode system permits safer implantation with reduced trauma to patients, posing an exciting new development compared to conventional methods of brain implants.
This pilot study primarily focuses on determining Synchron's BCI device's safety and effectiveness in individuals with severe paralysis. The breakthrough capability of capturing and relaying brain signals that enable the thought-controlled operation of digital devices through their Stentrode system stands poised to revolutionize how such patients interact with their environment. It presents unprecedented opportunities for those afflicted by substantial mobility limitations to reclaim aspects of independence and substantially enhance life quality.
The pioneering work by Synchron in advancing BCI technologies marks a pivotal progression within this scientific domain. By significantly lowering barriers associated with implant procedures, it extends potential benefits more broadly across affected populations needing support most desperately. With this inaugural U.S.-based implementation, there is tangible promise reflected by Sychron's BCI efforts. They serve not just as technical achievements but beacon-like symbols igniting possibility — potentially reshaping day-to-day existence among those wrestling against challenges brought forth through severe paralysis conditions.
Clinical Research and Safety Measures
Synchron's clinical investigation is rigorously structured to validate the safety and effectiveness of its BCI device. The objective of the clinical trial is to evaluate how well the device enables patients to manage everyday digital activities independently. Six patients' enrollment in this trial represents a notable advancement in brain-computer interface technology.
Synchron has achieved a significant breakthrough in its U.S. feasibility study by successfully implanting its BCI device in six individuals, underscoring significant strides within this domain. After a 12-month monitoring period, results are anticipated, promising crucial evidence regarding these devices' enduring safety and operational efficiency. Evidence from one-year observations confirms no deterioration in signal strength, which is essential for continuous enhancement efforts.
An up-and-coming discovery from the study was the absence of serious adverse events stemming from implanting the apparatus. This discovery affirms confidence in Synchron's endovascular methodology as a harmless and effective procedure for embedding brain-computer interfaces through neuro-interventional means. Ensuring patient safety during insertion into cerebral vasculature remains at the forefront of research priorities.
BCIs present a form of communication independent of muscle function, especially beneficial for those afflicted with advanced ALS stages where conventional methods become ineffective due to motor control degradation. They offer viable alternatives that enable expression despite loss of muscular voluntary action, thus improving life quality markedly for such individuals.
Through steadfast dedication towards rigorous studies prioritizing security measures, Synchron is laying the groundwork conducive to actualizing enhanced autonomy and command over personal environments amongst people grappling with severe motion disabilities.
Successful Trials in Australia
The Synchron BCI device was tested with ALS patients in Australia before the trials in the United States. The trial involved four patients and showed promising results, indicating that the technology could improve the quality of life for those with severe motor disabilities.
These encouraging outcomes from Australia provided a solid basis for U.S. trials, underscoring the worldwide influence of Synchron's cutting-edge device on patient care.
Mechanism of Synchron's BCI Technology
Synchron's BCI technology is an extraordinary integration of neuroscience and engineering prowess. It employs a dedicated electrode array designed to capture motor signals inside blood vessels through an endovascular approach, which enhances safety and accessibility compared to conventional open-brain surgical methods.
The technique provides direct access to the brain's motor cortex, facilitating the effective transmission of neural signals while substantially diminishing the hazards that typically accompany traditional surgical interventions.
Stentrode System
The Stentrode system is a key component of Synchron's BCI technology, designed to be implanted via blood vessels, specifically the jugular vein. This minimally invasive delivery method allows direct access to the brain's motor cortex, enabling the capture and transmission of brain signals without opening brain surgery. The ability to safely implant the device through the vascular system represents a significant advancement in neuroprosthetics.
Once implanted, the Stentrode device can capture motor signals from the brain and translate them into digital switches, allowing users to control digital devices. This technology opens up new possibilities for individuals with severe motor impairments, enabling them to interact with their environment and perform previously impossible tasks.
The Stentrode system's minimally invasive nature and its ability to capture brain signals make it a revolutionary tool in the quest to restore independence to those with severe disabilities.
Neural Signals and Digital Devices
The Stentrode system's ability to capture neural signals from the motor cortex and translate them into digital switches is a game-changer for individuals with restricted physical abilities. Placing the electrode array inside a blood vessel on the brain's surface allows the device to effectively capture brain motor signals, facilitating control over digital devices. This technology allows users to perform online banking and control smart home devices, significantly enhancing their independence and quality of life.
The Synchron device processes neural signals from the motor cortex, enabling users to control digital devices with their thoughts. This breakthrough has profound implications for individuals with severe motor impairments, providing a new method for interacting with technology without relying on muscle movement. The device can generate digital switches from neural signals, empowering users to engage in activities that were once beyond their reach.
The potential applications of this technology are vast, ranging from communication tools to smart home devices. For example, patients can use the device to send messages, control lighting, and temperature in their homes, and even manage their finances through online banking.
The ability to translate brain signals into digital commands represents a significant step forward in improving the quality of life for individuals with severe disabilities.
Minimally Invasive Procedure
One of the most remarkable aspects of Synchron's BCI technology is the minimally invasive endovascular procedure used to implant the device. Accessing the motor cortex through the jugular vein bypasses the need for open brain surgery, significantly reducing the potential risks associated with traditional surgical interventions. This approach makes the procedure safer and more accessible for patients with limited physical mobility.
The endovascular method allows for the safe implantation of the BCI device, enabling patients to regain control over their environment and perform tasks independently. Avoiding the complications of open brain surgery, this minimally invasive procedure represents a significant advancement in neuroprosthetics, offering new hope for individuals with severe motor impairments.
FDA and Investigational Device Exemption
Regulatory bodies have taken note of Synchron’s progress in the field. The company has been awarded an investigational device exemption by the Food and Drug Administration for its brain-computer interface that is meant to be permanently implanted. This significant step, approved under FDA guidelines, permits Synchron to conduct tests on the safety and performance of its BCI technology as it works towards broader accessibility. Within this context, the National Institutes of Health’s BRAIN Initiative supports a clinical trial to determine if patients with significant mobility limitations can operate digital devices through their thoughts using the Synchron Switch neuroprosthesis.
The study enrolled six individuals who suffer from severe quadriparesis. These participants underwent a neuro-interventional procedure to have the device fitted within them. A key aspect being evaluated during this clinical investigation is any potential adverse events associated with using this novel treatment option, which will be systematically documented as required by ClinicalTrials.gov.
By diligently tracking outcomes throughout this experiment, Synchron seeks evidence that could underscore just how much their BCI technology might revolutionize lives—specifically aiming at bettering independence and well-being for those facing severe physical challenges—with hopes pinned on successful control over computer interfaces directly via cerebral implantation.
Future Implications for Degenerative Diseases
The implications of Synchron’s BCI technology extend far beyond the current clinical trials. For individuals with degenerative diseases such as Amyotrophic lateral sclerosis (ALS), the ability to control personal devices using neural signals represents a significant breakthrough. Synchron’s BCI technology interprets neural signals to operate electronic devices, enhancing user independence and providing a new avenue for communication and interaction.
The trials in Australia demonstrated that participants could regain independent control over technology, significantly enhancing their daily activities. Patients used the device to perform tasks such as online shopping and managing finances, showcasing the technology's practical applications. Mark, a participant with ALS, expressed that being involved in the trial felt like a clear choice driven by the lack of available treatments for his condition. His journey highlights the emotional struggle of losing independence and the hope BCI technology can bring.
Through the use of the BCI, Mark has experienced a renewed sense of hope for himself and the potential to assist others with similar conditions. The deployment of BCIs in clinical settings may require new protocols to address the unique needs of ALS patients, particularly concerning their communication capabilities.
The future of BCI technology holds great promise for individuals with degenerative diseases, offering new possibilities for independence and improved quality of life.
Patient Experiences and Testimonials
The experiences of individuals participating in clinical trials are a testament to the effectiveness of Synchron’s technology. Philip O’Keefe, for example, became a trailblazer when he used only his thoughts and a BCI device to post a tweet. This event marked significant progress in practical applications for people with severe disabilities who wish to communicate.
Another trial participant, Mark, showed remarkable progress after being implanted with the Stentrode. It took him about two months following surgery to adeptly navigate control using the device. He described feeling like his brainwaves were flexing (expanding and contracting), aligning with his mental commands during training sessions. His ability has evolved to include sending health updates and aspiring towards texting or managing smart home technologies through thought alone.
By capturing motor intent-related brain signals, this revolutionary BCI technology gives patients command over digital tools solely via neural activity. The functionality reaches far enough to allow them not just simple interaction but actual control over their smart homes and communication devices, significantly augmenting their capacity for autonomy within their environment while maintaining social connections without physical movement or speech.
Patient stories such as these underscore how transformative Synchron’s innovation is by empowering those with profound disability constraints. It stands out because it furnishes users not merely assistance but near-normal interactions between themselves and the world around them—a leap forward into personal empowerment that brings restored independence and renewed hopefulness.
Summary
Synchron’s advancements in brain-computer interface technology represent a significant neuroprosthetic leap forward. The successful completion of the COMMAND trial, the first U.S. implantation of the BCI device, and the comprehensive clinical research conducted highlight the potential of this technology to transform the lives of individuals with severe motor impairments. The positive results from both the U.S. and Australian trials underscore the efficacy and safety of Synchron’s device, paving the way for its broader application.
As we look to the future, the implications of Synchron’s BCI technology for degenerative diseases such as ALS are profound. By enabling individuals with severe paralysis to control personal devices using neural signals, Synchron offers a new level of independence and quality of life. The inspiring testimonials from patients like Philip O’Keefe and Mark illustrate the real-world impact of this technology, providing hope and new possibilities for those with severe disabilities. The future of BCI technology is bright, and Synchron is at the forefront of this transformative journey.
Frequently Asked Questions
What is the primary goal of Synchron's COMMAND trial?
The COMMAND trial’s foremost objective is to evaluate the safety and effectiveness of the Synchron Switch motor neuroprosthesis, which aims to empower individuals suffering from severe paralysis with the ability to carry out daily tasks independently.
How does Synchron's BCI device get implanted?
The BCI device from Synchron is inserted through a minimally invasive method through the jugular vein. This allows for direct connection to the motor cortex and reduces risks associated with typical brain surgeries.
What are some of the safety measures taken during the clinical trials?
Clinical trials establish thorough safety protocols, which include a 12-month follow-up period to meticulously track the device's performance and detect any serious adverse events.
These measures guarantee the safety of participants throughout the entire duration of the clinical trial.
How does the Stentrode system work?
Implanted via the blood vessels, the Stentrode system taps into the motor cortex to harvest brain signals. It then translates these signals into digital commands, allowing individuals to manipulate digital devices solely through mental commands.
What impact has Synchron's BCI technology had on patients?
The BCI technology developed by Synchron has notably improved the autonomy and quality of life of patients by enabling them to better communicate and engage with their surroundings. This is evidenced by users such as Philip O’Keefe and Mark, who have experienced these enhancements firsthand.
Thanks to this breakthrough, individuals can now compose messages and manage smart home appliances.
Further Reading